Postpartum Depression Gets a Fast-Acting Fix
Deep emotional distress after birth kills many mothers. A new kind of drug offers better, faster treatment

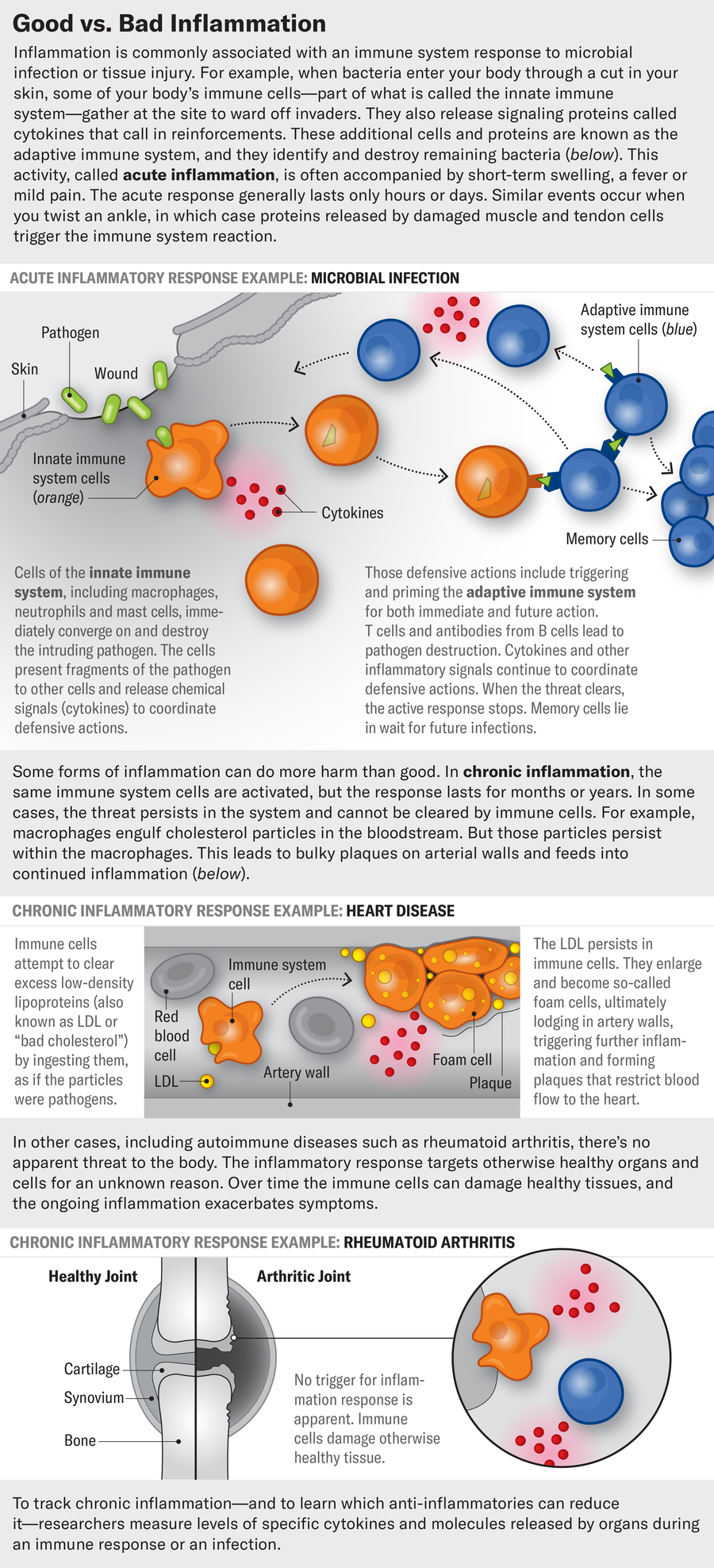
Kristina Leos (left), who went through severe depression after the birth of her daughter Victoria, leans in to kiss her child.
Deep emotional distress after birth kills many mothers. A new kind of drug offers better, faster treatment
Postpartum depression descended on Kristina Leos like a heavy fog that separated her from everyone she loved. She could see her newborn baby girl, her two older kids and her husband, but she felt like a ghost passing through their world. "I was going through the motions, but it was like I was looking down on my family," she recalls.
Leos, 40, a nurse who lives in Midlothian, Tex., tried several different antidepressants and doses. None helped. She messaged a friend, anxious that she was unfit to be a mother. She even asked if they would take her new baby, Victoria. Although Leos never considered hurting her kids, there were times when she was driving home from work and wondered what it would be like to drive off a bridge. "I just had no fear of dying," she says. "I didn't care what happened."
In December 2023, nine months after Leos gave birth to Victoria, her doctor told her they were running out of options. She was down to serious choices, including infusions of ketamine (a drug that alters the anatomy and activity of brain cells), electroconvulsive therapy or admission to a psychiatric hospital.
Then Leos remembered seeing something on social media about a new drug specifically for postpartum depression. Unlike older antidepressants such as Prozac, this medication worked on brain chemicals that are particularly affected by pregnancy. She asked her doctor about it, and they decided to give it a try. Leos began the medication on New Year's Day 2024. Three days later her world shifted. "I was driving on the highway, and I could literally feel this huge cloud lifting over me," she says. "And every day I got better and better." The drug, called zuranolone and approved by the U.S. Food and Drug Administration in 2023, has since relieved depression in thousands of women.
This kind of help is needed desperately. For new mothers, the overall leading cause of death during the first year after childbirth is not bleeding or infection, according to one study encompassing 36 states. What kills more are mental health problems, which account for approximately 23 percent of maternal deaths in the country. These disorders include a lot of cases of postpartum depression. Yet fewer than half of the women who show signs of such illness are diagnosed, and even fewer receive any form of treatment.
Emerging research on the biology of postpartum depression shows that it is not like other severe mood disorders neurologically or biochemically. Rather it is a result of dramatic changes in hormone levels that come with pregnancy and childbirth. Studies have shown that levels of progesterone and a related hormone, allopregnanolone, rise significantly during pregnancy. Then the levels drop sharply after delivery. Some women are particularly sensitive to this drop, which can disrupt the brain circuitry that regulates mood, leaving them unable to effectively deal with the stresses of motherhood. Zuranolone is designed to offset that drop-off.

Leos finally got relief from her postpartum depression with a new medication, zuranolone; she felt better within days of her first dose.
Growing knowledge of the neurobiology of postpartum depression is also pointing toward methods for earlier and more reliable detection. Many experts hope that identifying biomarkers that predict which women will develop the condition, as well as the introduction of the new medication, will take the stigma away from the illness and stop both health-care workers and patients from viewing it as a sign of personal weakness or poor parenting. "It is a serious mental illness," says Kristina Deligiannidis, a reproductive psychiatrist at the Feinstein Institutes for Medical Research at Northwell Health in New York State. "We just want to empower women to seek treatment."
Challenges do remain. The price tag for the two-week course of zuranolone is nearly $16,000, raising concerns about how insurance coverage and looming Medicaid-eligibility cuts could restrict access, especially because Medicaid covers about 40 percent of births in the U.S. And researchers are still trying to figure out why the pill doesn't work for everyone. "Not every single person that takes it is going to have a fabulous remission of their symptoms," says Samantha Meltzer-Brody, a psychiatrist and founder of the perinatal psychiatry program at the University of North Carolina School of Medicine in Chapel Hill. Still, she views the medication as a major milestone. "It can work remarkably well for more than half of people, and it's rapid-acting," she says. "That's a game changer."
For centuries medicine has struggled to fully grasp the causes and consequences of postpartum depression. Descriptions go as far back as ancient Greece: physicians wrote about women who showed signs of a depressed mood, and even psychosis, after childbirth. During the Middle Ages new mothers with depressive symptoms were often believed to be possessed by demons or suffering from an imbalance of bile or other body fluids. Postpartum mood disturbances have also been grouped into vague or broad diagnoses such as melancholia, mania or neurosis, which did little to help patients.
Even in modern times, such distress is often dismissed as "baby blues"—the mood swings that affect most new moms but typically resolve within a couple of weeks. But postpartum depression is more intense and long-lasting. It can cause profound sadness and despair, disrupting the crucial bond between mother and child, and its consequences can affect multiple generations. Every year approximately 500,000 women in the U.S. experience the condition. Approximately 30 percent of women with postpartum depression continue to experience symptoms one year after giving birth. For some these problems can persist for as long as 11 years.
Yet postpartum depression is not officially recognized as a standalone illness. It did not appear in the Diagnostic and Statistical Manual of Mental Disorders (DSM), the so-called bible of psychiatry, until 1994. Even then it was listed as a subtype of major depression. In the most recent major edition, DSM-5, released in 2013, it is still subsumed under the "major depression" label, with the added phrase "with peripartum onset." These additional three words reflect evidence that almost half of women develop symptoms during pregnancy, not just after.
Because postpartum depression has been lumped in with major depression, the two have often been treated the same way. Therapy has relied on traditional antidepressants such as selective serotonin reuptake inhibitors (SSRIs) or serotonin and norepinephrine reuptake inhibitors. This approach is rooted in the idea that depression stems from low levels of chemical messengers such as serotonin and norepinephrine that help to govern mood. These antidepressants aim to boost levels of these messengers in the brain.
Not everyone who takes zuranolone is going to have a fabulous remission. Still, it works well for more than half the people. That's a game changer.
But in recent decades the research community has recognized that focusing only on these chemical imbalances leaves out other factors that may underlie postpartum depression—including genetics, inflammation, hormonal changes, and neuroplasticity, the brain's ability to adapt and form new connections.
Some scientists suspected that fluctuations in hormones such as estrogen and progesterone—called neurosteroids because they act in the brain—played an important role. Yet when research groups started examining the levels of various hormones and neurosteroids, they did not see consistent differences that explained why some new mothers developed depression and others did not.
Then, about 17 years ago, Jamie Maguire, a neuroscientist now at Tufts University, stumbled on some unusual behavior in mice that had just given birth, and her observation helped to connect the dots. At the time, Maguire was a postdoctoral fellow at the University of California, Los Angeles, studying an ailment called catamenial epilepsy, in which brain seizures become more frequent or more severe during certain phases of the menstrual cycle. She was interested in how neurosteroids might protect against these seizures. Some neurosteroids have been shown to dampen brain activity by strengthening certain effects of a neurotransmitter called gamma-aminobutyric acid, or GABA. This chemical can inhibit neurons, making them less likely to fire. Maguire genetically engineered mice to have altered receptors for GABA on their neurons, making it hard for them to react to the chemical. Without this "brake" on neural activity, the mice's brains became hyperexcitable. That extreme state can contribute to seizures.
But when Maguire tried to breed the modified mice, she noticed something unexpected. The new mothers showed strikingly poor maternal behavior—symptoms that, in rodents, looked an awful lot like depression.
"They deliver normally, but then during the postpartum period they fail to take care of their offspring, and a lot of [the babies] would die from neglect," Maguire says. Until they gave birth, the mice seemed perfectly healthy. "It's really something happening during this pregnancy and postpartum period that's eliciting these behavioral abnormalities," she explains. When Maguire gave the mice a compound that restored their ability to react to neurosteroid signals, they behaved as normal mouse mothers did, and more pups survived.
This discovery led to a slew of studies investigating how neurosteroids affect vulnerability to postpartum depression, as well as a new theory for how childbirth can trigger mood disorders. During pregnancy neurosteroids surge to extremely high levels—up to 100 times higher than in a typical menstrual cycle—to help the body prepare for the physiological and psychological demands of motherhood. Maguire showed that to handle this flood of hormones the brain reduces the number of GABA receptors in certain regions. This adjustment helps to prevent bothersome and sometimes dangerous symptoms such as severe drowsiness. But those hormone levels drop precipitously at delivery, leaving the brain in a precarious position.
Typically brain cells sense this shift and dial the receptors back up over the course of several weeks, and all is well. But "if you fail to recover those receptors, you get this vulnerability for mood disorders," Maguire says.
This vulnerability arises because the body's stress-response system, known as the hypothalamic-pituitary-adrenal (HPA) axis, gets thrown off-kilter. When the body senses stress, it unleashes a cascade of signals: the hypothalamus sends a message to the pituitary gland, which then tells the adrenal glands to release cortisol and later adrenaline, hormones involved in the body's fight-or-flight responses. Maguire says this reaction is usually blunted during pregnancy and immediately after childbirth because of rising levels of neurosteroids and the activity of GABA. These substances dampen HPA-axis activation so mothers can bond, quietly and peacefully, with their little ones. But if that suppression continues for too long, postpartum depression symptoms start to appear.
Psychiatrist Kristina Deligiannidis says "we had women in the studies who wanted to die." Yet after treatment the self-destructive thoughts disappeared.
Brain-imaging studies suggest that treatment with neurosteroids can restore healthy communication among these various neural pathways and the large-scale networks that connect them, allowing the maternal brain to respond appropriately to stress. "We think that the antidepressant effects of these neurosteroids involve the ability to kind of reset these network states," Maguire says.
A few years after Maguire created her first melancholic mouse models, neuroscientist and pharmaceutical executive Steve Paul co-founded a company called Sage Therapeutics to develop neurosteroid-based medicines for brain disorders. Paul once served as scientific director of the National Institute of Mental Health, where he showed that the neurosteroid allopregnanolone quieted overactive neurons. It did so by modulating their GABA receptors. Allopregnanolone appeared to be a promising way to control neuron behavior.
In 2012 Sage Therapeutics began clinical research on a synthetic form of allopregnanolone called brexanolone that could be given to patients intravenously. The company, working with outside collaborators such as Meltzer-Brody of U.N.C., ran exploratory studies for an involuntary shaking disorder called essential tremor and for postpartum depression. In one small study, Meltzer-Brody gave four women with severe postpartum depression a 60-hour infusion of brexanolone. The experiment did not have a placebo control, making it difficult to determine whether the treatment was truly effective. Still, "the findings of that study were just jaw-dropping," says Deligiannidis, who was not involved in this initial work. Every one of the four women experienced such a remarkable recovery that they no longer met the criteria for clinical depression.
Three larger clinical trials followed, each led by Meltzer-Brody, and they did have placebo controls. In total, 267 women with postpartum depression received either brexanolone or a placebo infusion. The majority of the women given brexanolone did better clinically, with at least 50 percent improvement on a test called the Hamilton Rating Scale for Depression. Even with a strong placebo effect—which often happens in depression studies—the results were impressive. For instance, in one high-dose brexanolone study, 61 percent of patients receiving the treatment went into remission, compared with 38 percent of those taking the placebo.
The work led to FDA approval of brexanolone in March 2019 as the first pharmacological therapy specifically indicated for postpartum depression. The picture was not all rosy, however. The trials also showed that the drug could cause women to feel dizzy or drowsy and in some cases even lose consciousness. Because of these issues, the medication required continuous medical supervision, creating an emotional and financial barrier for many patients. "They would have to check into a clinic and be there for 60 hours for the infusion," says Benjamin Bruno, vice president of clinical development at Lipocine, a Salt Lake City–based drug-delivery company specializing in hormones and neurosteroids. "This drug, it works great, but no one's using it because it's IV."
Michael Quirk, former chief scientific officer at Sage Therapeutics, says the company recognized that an oral drug would be the best way to treat patients with postpartum depression. The trouble, he says, is that naturally occurring allopregnanolone—the active ingredient in brexanolone—has poor oral bioavailability; less than 5 percent gets into the bloodstream if given by mouth. So scientists set about tweaking it and eventually created an effective orally delivered compound that retained a lot of brexanolone's GABA-enhancing action.

After Victoria's birth, Leos desperately worried that she would not be able to take care of her youngest daughter.
The result, zuranolone, was not simply an oral version of brexanolone. "It's a completely distinct new chemical entity—until Sage chemists made it, it never existed anywhere in the world," Quirk says. (He is no longer with Sage, which was bought out by a pharma company called Supernus in 2025.) The new molecule worked. In one study, 153 women with severe postpartum depression were randomly selected to take either zuranolone or a placebo pill every evening for 14 days. The women started off with scores of about 28 out of 52 on the standard Hamilton depression scale, the same one used to evaluate brexanolone in earlier work. By the end of the study, the zuranolone group's scores had dropped to around 9, whereas the placebo group's scores averaged about 14. The antidepressant effects were rapid, with patients experiencing symptom relief in as few as three days. And they were sustained, with patients continuing to report fewer depressive symptoms even after the medicine had left their system.
Deligiannidis, who led this clinical trial, says she will never forget the transformation she witnessed. She recalls that many of the women struggled with the most basic daily tasks—brushing their teeth, taking a shower, even getting out of bed. They had little to no appetite, often surviving on coffee to stay alert, and they poured what little energy they had into caring for their baby. "We had women in the studies who wanted to die; really their hopelessness was at a point where they believed they were burdens to their family," she says. Yet after treatment those self-destructive thoughts disappeared for many. The medication "can be a lifesaving intervention."
The FDA approved zuranolone in the summer of 2023, just before Leos reached her lowest point and thought she was running out of options. She was nervous about taking a drug that had just arrived on the market, and she obsessed over the medication instructions. "I read that front to back so many times, the side effects and how to take it," she says. For her, some dizziness and sleepiness were tolerable. If anything, the meds helped her finally get a decent night's sleep. "[Before] I would just wake up in the middle of the night anxious about things, and I could never sleep," she remembers.
The American College of Obstetricians and Gynecologists now recommends zuranolone as a treatment option. Camille Meehan, an obstetrician-gynecologist in Tulsa, Okla., says most of the women with postpartum depression she sees have moderate to severe cases because those with mild depression might not seek medical help. Meehan says she offers zuranolone as well as traditional SSRI antidepressants to her patients, talking through the risks and benefits of each. For example, SSRIs can take four to six weeks to reach full effect, whereas zuranolone often works within days. A full course of zuranolone takes two weeks. The speed is attractive. "It's hard not to use it as a first-line treatment when you know this mom is going through this acute period that can escalate quickly," Meehan says. Yet women's experiences with the new medication have varied widely, she tells her patients. Some people show dramatic improvement, whereas others see only modest or short-lived benefits. Some stop early because of side effects such as drowsiness.
In clinical studies, about 60 percent of patients had a meaningful reduction in depressive symptoms. (For context, traditional SSRIs work for about 50 to 60 percent of people with other types of depression who take them.) Around 16 percent reduced their dose because of side effects, and about 4 percent stopped taking the drug entirely. Currently there's no reliable way to predict who will respond and why, although Meltzer-Brody says the different outcomes suggest different underlying mechanisms are at play. "I think what we've come to appreciate is there's not one kind of postpartum depression—there are likely many different kinds," she says. "It just, again, speaks to the need for ongoing science and development."
Changes linked to two genes may predict the likelihood of someone developing postpartum depression.
Zuranolone may be the beginning of a new generation of medications for postpartum depression, although the number of players is small and the funding is limited. Lipocine, for instance, is using a proprietary lipid technology to develop new oral versions of the older drug, brexanolone. And Taiwan-based TWi Biotechnology is developing NORA520, an oral "prodrug" that gets converted into brexanolone in the body.
Yet even with a pill for postpartum depression on the market and others on the near horizon, many women continue to suffer. That's why researchers are searching for biomarkers to identify women who are at risk and predict who is most likely to benefit from new treatments.
For example, reproductive psychiatrists and longtime collaborators Jennifer L. Payne of the University of Virginia and Lauren M. Osborne of Weill Cornell Medicine in New York City have measured levels of various neuroactive steroids—all related to progesterone, such as allopregnanolone—to see how they relate to postpartum depression risk. They found that women who developed the condition had distinctive hormone patterns in the third trimester of pregnancy. Their pregnanolone-to-progesterone ratio was lower than that of women in whom the illness did not arise, and their isoallopregnanolone-to-pregnanolone ratio was higher.
These discoveries are important clues, but Payne says coming up with a test based solely on circulating neurosteroid levels will be difficult. The hormones fluctuate naturally, and the differences tend to show up as trends within groups rather than as red flags in individual patients. Still, the findings suggest something is shifting biologically before any mood or emotional symptoms appear. And they raise a key question: Do these signals in the blood truly reflect what is happening in the brain?

Having survived the family crisis, Victoria, her mother, her brother, Joseph, and her sister, Eileen (clockwise from top), are enjoying time together near their home in Midlothian, Tex.
That is where a newer type of biomarker comes in. It is based on extracellular vesicles (EVs), tiny sacs, released by cells, that carry genetic material such as messenger RNA (mRNA) throughout the body, along with other molecules. Because some of these mRNAs originate in the central nervous system, EVs offer a potential window into what is happening in the maternal brain. Sarven Sabunciyan, a neuroscientist at Johns Hopkins University, discovered that the mRNA content of EVs in maternal blood was extensively altered during and after pregnancy in women who developed depression. In particular, he found a dearth of mRNAs involved in autophagy, cells' waste-removal system. "Autophagy is actually disrupted in neurodegenerative disease," Sabunciyan says. "And there's evidence for it in psychiatric disease—I don't think we've done enough of a deep dive, but that's what our data are pointing toward." Sabunciyan is optimistic that tests that use EV-based biomarkers will be feasible within a decade or so.
In the nearer term, promising clues for identifying postpartum depression come from a field known as epigenetics. Epigenetic changes, such as the addition of chemical groups called methyl tags to DNA, change the quantities of proteins that affect the body's stress response. A team led by Payne and Osborne identified DNA-methylation changes in two genes, called HP1BP3 and TTC9B, that seem to predict who is likely to get postpartum depression. Not coincidentally, both genes have been linked to neurons' sensitivity to estrogen and thus to reproductive hormonal changes.
All of this biological discovery is helping to reframe postpartum depression as not an inevitable emotional struggle but a treatable condition with clear roots in the brain. But with the advent of zuranolone, treatment hopes were accompanied by fears that insurers would balk at a therapy that costs $8,000 per week—keeping healing out of reach for many. Since then, however, all major commercial insurers have put formal coverage policies in place, and most cover the medication without burdensome restrictions. So do state Medicaid programs. A financial assistance program from the manufacturer provides the drug at no or reduced cost to eligible patients.
Still, a few states—including Alabama, Alaska, Mississippi and North Carolina—require patients to try other antidepressants and show those drugs failed before they will cover zuranolone. Prior authorization is still the norm in these and other places. Many physicians say jumping through hoops to get administrative approval can be frustrating. Meehan, the OB-GYN in Oklahoma, says the approval process is worse with some insurers, delaying treatment.
Systemic inequities can also prevent access, not only to new medication but to all forms of postpartum mental health care. Many women, especially those in rural areas and in communities of color or those without stable insurance, can face significant barriers, from provider shortages to financial constraints. On top of that, stigma surrounding postpartum depression often keeps women from seeking help.
But if the shift to viewing postpartum depression as a biological disease continues, Meehan says, "that would be huge." She says having new ways of diagnosing and treating the condition could provide a starting point for providers to talk with women who may feel uncomfortable or stigmatized about what they are experiencing. "That is going to allow us to have a conversation, kind of guide it in a little bit of a different direction."
Leos says that in her job as a neonatal intensive care nurse, she often recognizes the signs of deep sadness in women she encounters. She makes time to sit and talk with them about how they are really feeling, remembering that she felt too ashamed after her youngest girl was born to be honest about her emotions.
She wants these other women to learn from her story and to get the help they need. "Postpartum depression robbed me of my baby's first year. I don't remember much about it," she says. "I don't really have any good pictures that showed me happy or throwing her up in the air, smiling." She's missing a good part of the past.
But Leos does realize that because she fought to find a solution, she and her entire family have the future. And, she says, "I'm very thankful for that."